National Sickle Cell Month on September, 2024: how many people get sickle cell in the united states?
September, 2024 is National Sickle Cell Month 2024. Sickle Cell Disease Association of America, Inc. Sickle Cell Disease
As an Amazon Associate I earn from qualifying purchases.

Sickle cell anemia is a hereditary disorder that mostly affects people of African ancestry, but also occurs in other ethnic groups, including people who are of Mediterranean and Middle Eastern descent. More than 70,000 Americans have sickle cell anemia. And about 2 million Americans - and one in 12 African Americans - have sickle cell trait (this means they carry one gene for the disease, but do not have the disease itself).
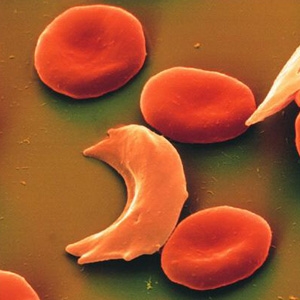
Sickle cell anemia occurs when a person inherits two abnormal genes (one from each parent) that cause their red blood cells to change shape. Instead of being flexible and round, these cells are more rigid and curved in the shape of the farm tool known as a sickle - that's where the disease gets its name. The shape is similar to a crescent moon.
What Is Sickle Cell Anemia?
Sickle cell anemia is a blood disorder that affects hemoglobin (pronounced: hee-muh-glow-bin), a protein found in red blood cells that helps carry oxygen throughout the body.
Red blood cells with normal hemoglobin (HbA) move easily through the bloodstream, delivering oxygen to all of the cells of the body. Normal red blood cells are shaped like doughnuts with the centers partially scooped out and are soft and flexible.
Sickle cell anemia occurs when an abnormal form of hemoglobin (HbS) is produced. HbS molecules tend to clump together, making red blood cells sticky, stiff, and more fragile, and causing them to form into a curved, sickle shape. Red blood cells containing HbS can go back and forth between being shaped normally and being sickle shaped until they eventually become sickle shaped permanently. Instead of moving through the bloodstream easily, these sickle cells can clog blood vessels and deprive the body's tissues and organs of the oxygen they need to stay healthy.
Unlike normal red blood cells that last about 4 months in the bloodstream, fragile sickle cells break down after only about 10 to 20 days, which usually causes anemia. Anemia (pronounced: uh-nee-mee-uh) is what happens when the body's number of red blood cells (or amount of hemoglobin) falls below normal. People who are anemic often feel weak and tire more easily.
People with sickle cell anemia can also experience complications from blood circulation and infection-fighting problems. These include a higher risk of certain infections and stroke as well as a condition called acute chest syndrome, which is caused by infection or trapped red blood cells in the lungs.
Sickle cell anemia is not contagious, so you can't catch it from someone else or pass it to another person like a cold or other infection. People with sickle cell anemia have inherited two sickle cell genes, one from each parent. A child who has inherited the sickle cell gene from only one parent will not develop the disease, but will have sickle cell trait. People who have sickle cell trait don't have sickle cell anemia or symptoms of the disease, but they can pass the sickle cell gene to their own children.
Because people with sickle cell trait don't have the disease, they may never discover that they carry the gene. That's why it's recommended that teens who are unsure of their sickle cell status ask their doctors about testing. The National Institutes of Health recommends that all newborns be screened for sickle cell disease, and testing at birth is now required in almost every state. This helps infants with sickle cell anemia get the care and treatment they need right away.
Is it possible for white people to have Sickle Cell Anemia?
I HOPE THIS HELPS....
Sickle cell anemia is a hereditary disorder that mostly affects people of African ancestry, but also occurs in other ethnic groups, including people who are of Mediterranean and Middle Eastern descent. More than 70,000 Americans have sickle cell anemia. And about 2 million Americans - and one in 12 African Americans - have sickle cell trait (this means they carry one gene for the disease, but do not have the disease itself).
Sickle cell anemia occurs when a person inherits two abnormal genes (one from each parent) that cause their red blood cells to change shape. Instead of being flexible and round, these cells are more rigid and curved in the shape of the farm tool known as a sickle - that's where the disease gets its name. The shape is similar to a crescent moon.
What Is Sickle Cell Anemia?
Sickle cell anemia is a blood disorder that affects hemoglobin (pronounced: hee-muh-glow-bin), a protein found in red blood cells that helps carry oxygen throughout the body.
Red blood cells with normal hemoglobin (HbA) move easily through the bloodstream, delivering oxygen to all of the cells of the body. Normal red blood cells are shaped like doughnuts with the centers partially scooped out and are soft and flexible.
Sickle cell anemia occurs when an abnormal form of hemoglobin (HbS) is produced. HbS molecules tend to clump together, making red blood cells sticky, stiff, and more fragile, and causing them to form into a curved, sickle shape. Red blood cells containing HbS can go back and forth between being shaped normally and being sickle shaped until they eventually become sickle shaped permanently. Instead of moving through the bloodstream easily, these sickle cells can clog blood vessels and deprive the body's tissues and organs of the oxygen they need to stay healthy.
Unlike normal red blood cells that last about 4 months in the bloodstream, fragile sickle cells break down after only about 10 to 20 days, which usually causes anemia. Anemia (pronounced: uh-nee-mee-uh) is what happens when the body's number of red blood cells (or amount of hemoglobin) falls below normal. People who are anemic often feel weak and tire more easily.
People with sickle cell anemia can also experience complications from blood circulation and infection-fighting problems. These include a higher risk of certain infections and stroke as well as a condition called acute chest syndrome, which is caused by infection or trapped red blood cells in the lungs.
Sickle cell anemia is not contagious, so you can't catch it from someone else or pass it to another person like a cold or other infection. People with sickle cell anemia have inherited two sickle cell genes, one from each parent. A child who has inherited the sickle cell gene from only one parent will not develop the disease, but will have sickle cell trait. People who have sickle cell trait don't have sickle cell anemia or symptoms of the disease, but they can pass the sickle cell gene to their own children.
Because people with sickle cell trait don't have the disease, they may never discover that they carry the gene. That's why it's recommended that teens who are unsure of their sickle cell status ask their doctors about testing. The National Institutes of Health recommends that all newborns be screened for sickle cell disease, and testing at birth is now required in almost every state. This helps infants with sickle cell anemia get the care and treatment they need right away.
What Can You Do to Stay Well?
With the right precautions, teens with sickle cell disease can do most of the stuff other teens do. To stay as healthy as possible, people with sickle cell anemia should take these steps:
Eat a balanced, healthy diet.
Take vitamins, including folic acid supplements, as prescribed.
Drink plenty of water to prevent dehydration.
Avoid extreme cold or heat.
Exercise regularly, but in moderation. Exercise is important for staying healthy, but overdoing it can trigger a crisis in some people, particularly if they become dehydrated, overheated, or exhausted.
Get plenty of rest.
Avoid alcohol, drugs, and smoking, which can aggravate sickle cell disease and its symptoms. Some people with sickle cell disease are prone to lung problems, so smoking is particularly risky.
Avoid places low in oxygen. (For example, it's not a good idea to go hiking at high altitudes or spend lots of time swimming under water.)
Prevent serious infections by contacting your doctor as soon as illness symptoms start. Be sure to get any immunizations (such as pneumonia and flu vaccines) that the doctor recommends.
Learn as much as you can about the disease and see your doctor regularly to help prevent complications.
There are some limits that people with sickle cell disease may need to put on their lives, but with the help of doctors, friends, and family, teens with sickle cell anemia can manage the disease and live their lives to the fullest.

Has anyone heard of the possible new sickle cell treatment Nicosan? What are the side effects?
Hope this helps, good luck
Nigerian scientists develop drug for sickle cell
By
Jul 6, 2004, 16:42
The National Institute for Pharmaceutical Research and Development (NIPRD), Abuja, has developed an anti-sickling drug for the management of sickle cell anaemia.
The drug, NIPRISAN, was developed from four local herbs and roots by the institute’s scientists nine years ago, but had to be subjected to a series of clinical trials which began in August 1995, NIPRD’s director General, Dr Uford S Inyang said.
At the end of the three-phased clinical trials conducted at the NIPRD Clinic, Abuja, NIPRISAN was found to be non-toxic and efficacious in the management of sickle cell anaemia.
According to Dr Inyang, “Phase I of clinical trials began in August 1995 here in NIPRD; Phase II followed in 2001, spanning 12 months with 81 sickle cell patients within the age range of 2 to 45; and Phase III has just been concluded.”
He said that the Institute has fulfilled the requirements for registration of NIPRISAN, “which means the drug will also go into the Nigerian market in July (2004).”
Dr Inyang also said that clinical trials for the drug are currently going on in four hospitals in the United States, “in order to meet the requirements for registration of the drug under US laws.”
The drug has been further upgraded and renamed NICOSAN by an American pharmaceutical company, Xechem International Incorporated.
Xechem’s Vice-President in charge of International Operations, Mr Bhuwan C. Pandey, explained that his company came to collaborate with NIPRD to upscale the drug to meet international standards.
“NICOSAN is entirely a Nigerian effort. Your scientists discovered the drug, which is actually an extract from four local plants”, he said.
Related Articles:
Anti-viral drugs to be produced in Nigeria
Nigeria to produce HIV/AIDS drugs
Researchers tackle AIDS drug resistance
© Copyright 2006 nigeriafirst.org
Nigerian President Olusegun Obasanjo Launches Xechem's Sickle Cell Drug, NICOSAN(TM), in Nigeria
Categories: Africa / Global / pharmaceutical / economy, business and finance / chemicals / health / Events / Events and Awards / ceremony / human interest / medical conditions and specialties / toxicology and pharmacology / government / public officials / politics / prescription drugs / science and technology / human physiology / health treatment / biology / United States
Recently in politics 2006/07/11:
Impeached Nevada Controller Dies
Cambodian gov't plans to privatize rubber plantations
Citizens offers extra coverage to meet code
Prized pay perk revoked for Collin Co. elected officials
Sales tax going up starting Saturday
Building safety sacrificed
New team for The Alhambra
NEW BRUNSWICK, N.J., Jul. 11, 2006 (Business Wire) -- Xechem International, Inc. (OTC BB:XKEM). Xechem International joins in congratulating its subsidiary, Xechem Pharmaceuticals Nigeria, on the successful launch of its new Sickle Cell drug, NICOSAN(TM), at a ceremony held on July 6th at Xechem Park, SHESTCO Complex, Abuja, Nigeria. The launching ceremony that was presided over by Nigeria's President, Chief Olusegun Obasanjo, was broadcasted live throughout Nigeria. Days earlier, the drug was approved by Nigeria's drug and regulatory authority, the National Agency for Food and Drug Administration and Control (NAFDAC). Xechem has obtained the exclusive worldwide rights to manufacture, market and sell NICOSAN(TM) under a licensing agreement with Nigeria's National Institute for Pharmaceutical Research and Development (NIPRD), a federal governmental agency whose scientists are credited with developing the drug.
President Obasanjo Tours Xechem Nigeria's Facilities
In remarks made at the launching ceremony, President Olusegun Obasanjo commended Xechem and its chairman, Dr. Ramesh Pandey, for their success to date in getting to the point of bringing this important drug to market and for validating the government of Nigeria's efforts to commercialize locally developed technologies: "In line with the reform agenda of this administration, the restructuring of the science, technology and innovation system has received priority attention. The re-engineering of the sector is hinged on the vision to make Nigeria a key participant and stakeholder in the application of new and emerging technologies and evolve an economy that is technology driven, private sector led and knowledge based. Today's occasion of launching of NICOSAN(TM) is an attestation that our policy is on the right course." Following the launching ceremony, President Obasanjo unveiled and toured Xechem's facilities and planted a tree on Xechem Nigeria's grounds to commemorate the occasion.
Dr. Pandey added: "We are extremely grateful to His Excellency, President Obasanjo, for making it a priority to personally launch NICOSAN(TM) and visiting Xechem's premises to mark this historic occasion. The President's participation in the ceremony reflects just how important this break-through sickle cell drug is, not only in Nigeria, but for those suffering with this debilitating disease all over the world." A gala affair attended by various ministers, high-level government officials, diplomats, and other business leaders, was held later that evening at the Abuja Sheraton Hotel in downtown Abuja.
About NICOSAN(TM)
NICOSAN(TM) is an anti-sickling "Natural Herbal Drug" developed by Nigerian Scientists at NIPRD and licensed by Xechem. In clinical studies conducted under NIPRD's auspices, the drug has shown to substantially reduce the degree of sickling of the affected red blood cells of those afflicted with the disease. While not a cure for SCD, the clinical trials have confirmed that the large majority of patients taking NICOSAN(TM) no longer experience sickle cell "crises" while on the medication, and even among those whose crises are not eliminated, the number and severity of the crises are substantially reduced.
About Sickle Cell Disease
Sickle Cell Disease (SCD) is an inherited blood disorder caused by an abnormality in the hemoglobin molecule. Patients with the disease often produce stiff, abnormally shaped red blood cells that often do not flow freely through the blood vessels. This can create clogs in the vessels, which in turn cut off the flow of normal hemoglobin and oxygen to parts of the body, and can cause severe painful attacks or "crises," damage to various organs and shortened life spans. People with SCD often suffer unpredictable painful crises several times a year lasting from a few hours to a week or more. In the US, there are approximately 80,000 patients with SCD. In Nigeria, that number is believed to be approximately 4 million, and worldwide at least 12 million individuals are afflicted with SCD.
About Xechem
Xechem International is a development stage biopharmaceutical company working on anticancer, antiviral (including AIDS), antifungal, antimalarial and antibacterial products from natural sources, including microbial and marine organisms. Its focus is on the development of phyto-pharmaceuticals (natural herbal drugs) and other proprietary technologies, including those used in the treatment of orphan diseases. Xechem's mission is to bring relief to the millions of people who suffer from these diseases. Its recent focus and resources have been directed primarily toward the development and launch of NICOSAN(TM) (to be marketed as HEMOXIN(TM) in the US and Europe). With the Nigerian regulatory approval now in hand, Xechem will now turn to the commercialization of the drug in Nigeria and the pursuit of US FDA and European regulatory approval. In addition to NICOSAN(TM), Xechem is also working on another sickle cell compound, 5-HMF, which it has licensed from Virginia Commonwealth University.
Forward Looking Statements
This press release contains certain forward looking statements within the meaning of Section 27A of the Securities Act of 1933 as amended, and section 21E of the Securities and Exchange Act of 1934, as amended, which are intended to be covered by safe harbors created hereby. Such forward looking statements involve known and unknown risks and uncertainties.
Newstex ID: BW-0001-9558733
Tagline:
Xechem International, Inc.
Stephen Burg, 707-425-8855
Credit: Xechem International, Inc.
Copyright (c) 2005 Business Wire. All of the news releases contained herein are protected by copyright and other applicable laws, treaties and conventions. Information contained in the releases is furnished by Business Wire's members, who warrant that they are solely responsible for the content, accurancy and originality of the information contained therein. All reproduction, other than for an individual user's personal reference, is prohibited without prior written permission.



















